Abstract
Background: Acute management of pain associated with vaso-occlusive episodes (VOE) in patients with sickle cell disease (SCD) is an on-going challenge in the emergency department (ED) setting. The 2014 National Heart, Lung, and Blood Institute (NHLBI) guidelines recommend rapid evaluation and treatment for moderate-severe VOE, including administration of 1stparenteral opioid within 30 minutes (min) of triage or 60 min from registration, pain reassessment, and repeat opioid delivery within 15-30 min. Despite these recommendations, significant delays in providing optimal pain management is common for patients with SCD. Intranasal fentanyl (INF), a powerful parenteral analgesia with rapid onset is commonly used for pain in the ED, a practice that has recently been extended to treatment of VOE in some institutions. We and others have demonstrated that INF use is effective in helping with sickle-related pain management and achieving better adherence to NHLBI guidelines. However, the utilization of INF may have led to an unanticipated delay in administering intravenous (IV) opioids for this patient population.
Objective: To evaluate the utilization of INF in the ED and its effect on the timeliness of 1st IV opioid administration for treatment of VOE in children with SCD.
Methods: We conducted a retrospective review of electronic medical records in a single Pediatric ED from January 2017 to June 2017. We evaluated patients with SCD ages ≥ 2 years presenting with moderate-severe VOE (pain scores ≥6 on a severity scale of 0-10) after introduction of a nurse-initiated INF order (1.5 mcg/kg/dose; max 100 mcg/dose X2, 5 min apart) that was added to the standard institutional protocol for VOE treatment. Outcomes included: Time of ED arrival to 1st parenteral opioid, time to first IV opioid, and disposition. We also compared time to 1st IV opioid administration in patients who received INF with historical data of SCD patients treated at this ED with only IV opioids before INF use was standard practice (Jan-Dec 2012, n=231).
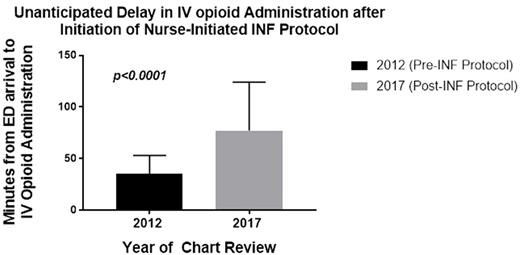
Results: 250 patients met inclusion criteria. Mean age was 12.5 years, 52% female, and 66% had HbSS, 22% HbSC, 6% HbSB0 and 6% other. Admission rate was 60%. 183 (73%) patients received INF and 204 (82%) received an IV opioid, 141 of whom received both INF and IV opioids, while 42 (17%) patients received INF only. Mean time±standard deviation to first INF dose was 28.5±21 min; mean time to IV opioid delivery was 77±47 min compared to 35±18 min prior to initiation of the INF protocol (historical data, n=231, p<0.0001; Figure 1). In the current cohort, there was no statistically significant difference in time to IV opioid delivery in patients who received IV opioid after INF vs. IV opioid alone. However, only 52 (36%) of patients who received IV opioid after INF received IV opioid within 60 min of ED arrival compared to 36 (57%) who received IV opioids without INF (p<0.01). Of the 42 patients who received INF without any IV opioids, 39 patients (93%) were discharged home, while 5 (13%) of these discharged patients returned to the ED within 72 hours for VOE, similar to the 72 hour return rate in 7/39 (18%) patients discharged from the ED after receiving INF followed by IV opioids.
Conclusion: Use of INF in the ED for pediatric patients with SCD and VOE is an excellent strategy to shorten time to administration of 1st dose of parenteral opioid and therefore improve adherence to the NHLBI guidelines recommending rapid administration of parenteral opioids within 60 min of ED arrival. Of interest, INF alone appeared to provide sufficient pain control without IV opioids for disposition home in one out of 6 children with VOE, an observation not previously reported. Whether this reflects adequate pain control or insufficient management requires further investigation. However, our experience also illustrates the potential pitfall of an ED-based INF protocol with respect to delays in delivery of IV opioids after initial administration of INF compared to our historical data prior to the INF protocol implementation. Although the significance of this delay is unknown, quality improvement efforts are underway to address this unanticipated consequence.
Morris: MAST Therapeutics: Research Funding; Calithera Biosciences: Consultancy; Nestle: Consultancy; Lifetrients: Patents & Royalties: I am inventor of IP owned by USCF-Benioff Children's Hospital Oakland, licensed to Lifetrients, generating royalties; Pfizer: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal